Molecular Characterization of Culturable Aerobic Bacteria in the Midgut of Field-Caught Culex tritaeniorhynchus, Culex gelidus, and Mansonia annulifera Mosquitoes in the Gampaha District of Sri Lanka
Abstract
Background
Larval and adult mosquito stages harbor different extracellular microbes exhibiting various functions in their digestive tract including host-parasite interactions. Midgut symbiotic bacteria can be genetically exploited to express molecules within the vectors, altering vector competency and potential for disease transmission. Therefore, identification of mosquito gut inhabiting microbiota is of ample importance before developing novel vector control strategies that involve modification of vectors.
Method
Adult mosquitoes of Culex tritaeniorhynchus, Culex gelidus, and Mansonia annulifera were collected from selected Medical Officer of Health (MOH) areas in the Gampaha district of Sri Lanka. Midgut lysates of the field-caught non-blood-fed female mosquitoes were cultured in Plate Count Agar medium, and Prokaryotic 16S ribosomal RNA partial genes of the isolated bacteria colonies were amplified followed by DNA sequencing. Diversity indices were used to assess the diversity and richness of the bacterial isolates in three mosquito species. The distribution pattern of bacterial isolates between different mosquito species was assessed by Distance-Based Redundancy Analysis (dbRDA).
Results
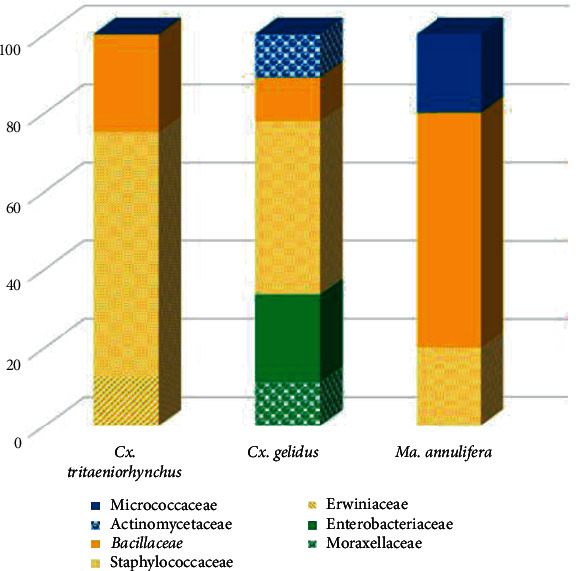
A total of 20 bacterial species (Staphylococcus pasteuri, Bacillus megaterium, Staphylococcus cohnii, Pantoea dispersa, Staphylococcus chromogenes, Bacillus aquimaris, Staphylococcus arlettae, Staphylococcus sciuri, Staphylococcus warneri, Moraxella osloensis, Enterobacter sp., Klebsiella michiganensis, Staphylococcus hominis, Staphylococcus saprophyticus, Streptomyces sp., Bacillus niacin, Cedecea neteri, Micrococcus luteus, Lysinibacillus sphaericus, and Bacillus licheniformis) were identified. All of these species belonged to three phyla, Proteobacteria, Firmicutes, and Actinobacteria, out of which phylum Firmicutes (71.1%) was the most prominent. The least number of species was recorded from Actinobacteria. The relative distribution of midgut microbes in different mosquito species differed significantly among mosquito species (Chi-square, χ2 = 486.091; df = 36; P ≤ 0.001). Midgut microbiota of Cx. tritaeniorhynchus and Cx. gelidus indicated a similarity of 21.51%, while Ma. annulifera shared a similarity of 6.92% with the cluster of above two species. The gut microbiota of Cx. tritaeniorhynchus was also significantly more diverse and more evenly distributed compared to Ma. annulifera. Simpson’s diversity, Margalef’s diversity, and Menhinick’s diversity indices were higher in Cx. gelidus. Of the recorded species, P. dispersa and strains of nonpathogenic species in Bacillaceae family (B. megaterium, B. niacini, B. licheniformis, and L. sphaericus) can be recommended as potential candidates for paratransgenesis.
Conclusion
The relative distribution of midgut microbes in different mosquito species differed significantly among the three studied adult mosquito species. The present data strongly encourage further investigations to explore the potential usage of these microbes through paratransgenic approach for novel eco-friendly vector control strategies.